by David Kordahl
 The photograph beside this text shows two men standing side by side, both scientific celebrities, both Nobel prizewinners, both of them well-known and well-loved by the American public in 1932, when the picture was taken. But public memory is fickle, and today only the man on the right is still recognizable to most people.
The photograph beside this text shows two men standing side by side, both scientific celebrities, both Nobel prizewinners, both of them well-known and well-loved by the American public in 1932, when the picture was taken. But public memory is fickle, and today only the man on the right is still recognizable to most people.
Albert Einstein, Time Magazine’s “Man of the Century,” the father of special and general relativity, has a place in science that remains secure, regardless of what one thinks of his life as a whole. Despite activist efforts at demystification, Einstein the scientist is unblemished by any misgivings about his personal life or political activities. Robert A. Millikan, the bow-tied man on the left, is far less secure. The posthumous charges against Millikan have been against his scientific integrity and his political sympathies, and his detractors have made headway.
In 2020, Pomona College changed the name of their Robert A. Millikan Laboratory, noting Millikan’s “history of eugenics promotion,” along with his purported sexism and racism. In 2021, the California Institute of Technology, the institution that Millikan spent decades building, followed suit, renaming Millikan Hall as Caltech Hall, and discontinuing the Millikan Medal, previously the Institute’s highest honor. Citing Caltech’s precedent, the American Association of Physics Teachers (AAPT) renamed its own Millikan Medal later that same year.
Since I spend most of my time teaching physics, and since I am myself a member of the AAPT, it was the last of these name changes that rankled me the most. These allegations bothered me because I suspected that they weren’t quite fair.
During the past few weeks, I’ve read through the cases against Robert Millikan. The rest of this piece will summarize these discussions. In the 1980s, the argument was made that Millikan had cherry-picked his data, but that conversation was sympathetic, and Millikan’s reputation emerged with only minor scuffs. The 2020s have been less forgiving to him, though Millikan still has some defenders.
Pessimistically, I concede that it’s probably unrealistic to suppose that Millikan’s reputation can be revived. This essay, then, acts as a sort of elegy, too late to make any difference.
Who was Robert Andrews Millikan?
If I feel a little protective toward Millikan, maybe it’s because, like me, he was an Iowan with little early exposure to physics. His start in physics, as Millikan himself recounted in The Autobiography of Robert Millikan (1950), suggests just how backward physics departments in the United States were at the time. Millikan was a classics major at Oberlin College and took physics during his sophomore year. He was then asked to teach physics during his junior year. “I was so intensely interested in keeping my knowledge ahead of that of the class that they must have caught some of my own interest and enthusiasm,” Millikan recalled.
Millikan spent four more years at Oberlin, during which time he continued to teach physics while also working as the director of the college gymnasium. When he arrived at Columbia University to pursue a Ph.D., he was the only graduate student in physics. After Columbia, Millikan spent a year in a German laboratory, where he managed to meet most of the figures now associated with the quantum revolution. Once he returned to the United States to take up a post at the University of Chicago, Millikan alternated between research and education, culminating in a series of physics textbooks that were widely used for decades.
Much more could be said about the Millikan biography. He was involved in the technical side of the American war effort during World War I, playing a pivotal role in the organization of the National Research Council. Afterward, Millikan moved from the University of Chicago to the Throop College of Technology—renamed as the California Institute of Technology when he arrived—and served as its president (or, rather, as its “chairman of the executive council”) from 1921 until 1945.
But the part of Millikan’s story that has recommended him to physics students for the past century involves two of his experiments, the two that were cited in his 1923 Nobel Prize, both of which are mainstays of the modern physics curriculum. His demonstration of the photoelectric effect, using filtered light from a mercury lamp, allowed for the best measurement of Planck’s constant at the time. And before that came the oil drop experiment—the subject of much later controversy.
Millikan’s oil drop experiment
The oil drop experiment is easy to understand, and painful to execute. What you observe through a microscope is just an oil droplet falling, then rising; falling, then rising. The version of this experiment that’s routinely carried out by students is a somewhat simplified version of Millikan’s original experiment, with somewhat less reliable results. (Fig. 1 shows the device I use with my students.)
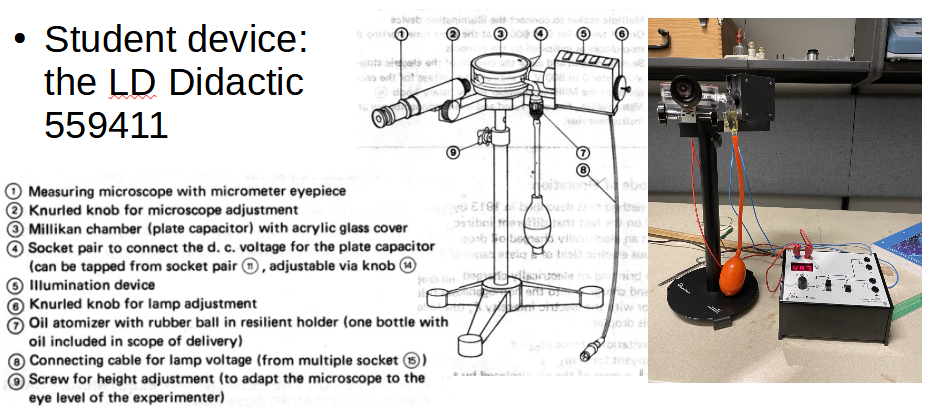
Let’s review this experiment again, now with more details. A light shines between parallel metal plates, which are connected to a voltage source that can be switched on and off by hand. The experimenter can spritz the region between these plates with an oil mist. The resulting droplets are very small, with diameters on the order of 1/1000th of a millimeter, and they quickly reach their terminal velocity, which can be measured by seeing how quickly they fall. When the voltage source is turned on, some droplets will change their directions, rising upward with different velocities than they once fell. These downward and upward velocities can be measured for many different oil droplets over the course of an afternoon, or, as with Millikan and his student, Harvey Fletcher, over the course of many months.
To make this clear, I have embedded a video I took recently by attaching my smartphone to the measuring microscope of the device shown in Fig. 1. More than one illuminated droplet, in this particular trial, can be seen changing its direction as the voltage source is turned on and off.
Such droplets, Millikan realized, each have a particular electric charge, proportional to how strongly the electric field from the applied voltage pulls the droplet upward. Readers curious about how the upward and downward velocities can be parlayed into charge calculations can consult an online textbook (here, for instance), but the upshot of this is just that each droplet’s charge can be found.
And so what? This experiment is classical in all its details, with techniques based in 19th-century physics, but it has a place in the development of quantum physics. This is because the charges Millikan found varied by specific amounts. Droplets possessed only integer numbers of electrons: 1 electron, 2 electrons, 3 electrons…and so on. Or, to wrap it in fancy lingo, Millikan found that charge is quantized.
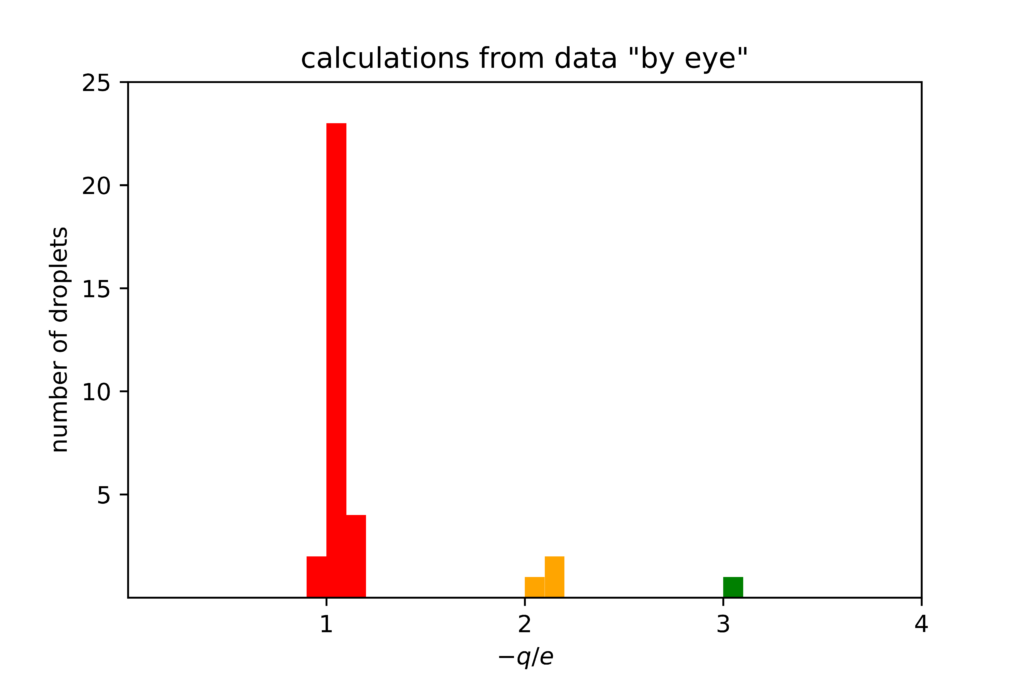
In Fig. 2, I’ve plotted some data I took last spring, using the apparatus shown in Fig. 1. I looked at 33 total droplets, which took a few hours. I measured with a stopwatch the time it took for each droplet to rise and fall over a set distance. The electron charge I found was systematically about 5% larger than the currently accepted value of the fundamental charge (denoted by “e”). Not too bad.
Scientific fraudster, or responsible experimentalist?
Results like those of Fig. 2 seem quite unambiguous, and Millikan, in his 1911 and 1913 papers, certainly presented his results that way. In The Electron (1917), Millikan wrote, “this is not a selected group of drops, but represents all the drops experimented upon during 60 consecutive days, no single one being omitted.”
But was this true? For his influential 1978 article, “Subelectrons, Presuppositions, and the Millikan-Ehrenhaft Dispute,” Gerald Holton examined Millikan’s original notebooks, and found that Millikan had observed many more droplets than were published. Indeed, it seems that Millikan had data for 140 droplets, and only included 58 of these in his papers. Millikan’s oil drop experiments had the rhetorical purpose, after all, of proving that charge was quantized, while other contemporaries—most notably, the Austrian physicist Felix Ehrenhaft, who conducted analogous experiments—argued against charge quantization.
Holton’s long paper is remarkably even-handed, and it does not vilify Millikan. The larger point Holton makes is that experimental scientists—especially those discovering phenomena whose basic contours are largely unknown—inevitably introduce theoretical assumptions when they judge which results are genuine, and which are the result of uncontrolled, accidental flukes.
There have been a few other notable reevaluations of the Millikan data. In the 1981 paper, “Millikan’s Published and Unpublished Data on Oil Drops,” Allan D. Franklin showed that, had Millikan included the excluded data, his average results for the fundamental charge would have hardly budged. Still, the idea that Millikan was thumbing the scale led him to be a featured sinner of “Honor in Science,” a pamphlet on research ethics published by the Sigma Xi scientific society in 2000.
The accusation by Sigma Xi led David Goodstein, a physics professor at Caltech, to revisit Millikan’s notebooks for his thorough apologia, “In Defense of Robert Andrews Millikan.” Goodstein noted that any experimentalist is likely to trust more strongly those results which he obtains after long experience with his equipment. In a clear chart, Goodstein shows that Millikan was much more likely to exclude measurements early on than he was as his experiment reached its end.
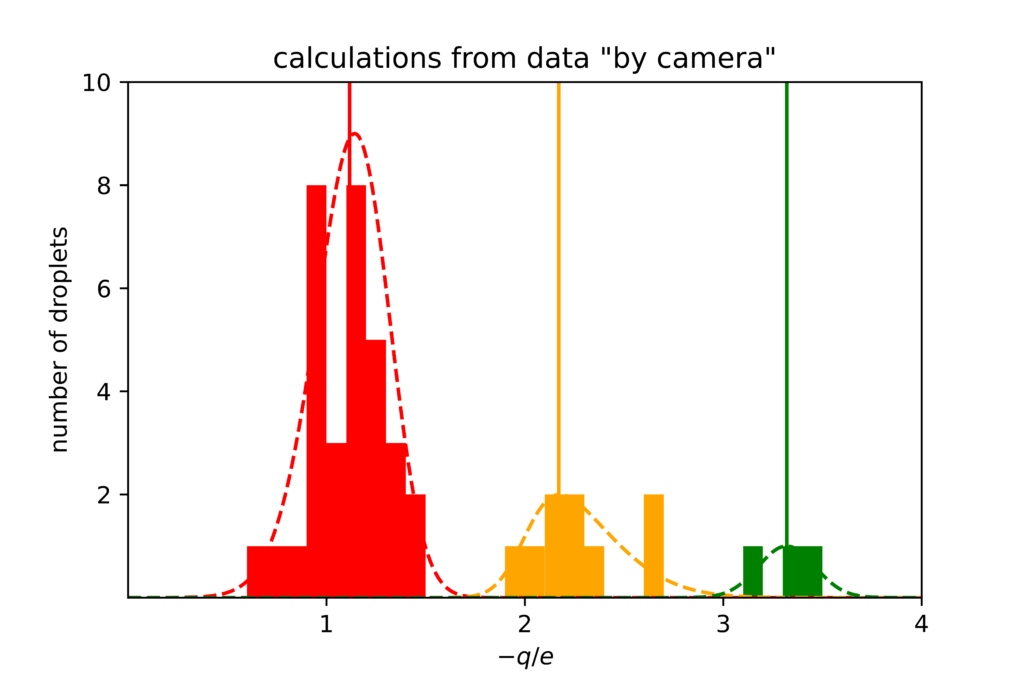
As a side note, I should admit that I recently tried Millikan’s experiment with “no single [drop] being omitted.” Of course, no data was intentionally omitted from Fig. 2, either, but selecting droplets that can be clearly seen and controlled is its own sort of omission. When I tried to analyze each of the 44 visible droplets that changed directions in twenty minutes of video footage (the video embedded above yielded three of these), the results were messy, with large systematic errors.
The de-Millikan-ization of Caltech, etc.
In his aforementioned “Defense,” David Goodstein commented that Millikan had been subject to criticisms of character that ranged beyond the purely scientific.
“In recent times,” wrote Goodstein in the year 2000, “Millikan has become a juicy target for certain historians because he was very much a part of the establishment, as well as being white and male, and, of course, he is no longer here to defend himself (I’m trying to fill in on that last point).” Goodstein quoted private communications where Millikan could be credibly be accused of antisemitism and misogyny, but his statements were “certainly not the rantings of a mindless bigot.” In his leadership of Caltech, Millikan attempted something like an absolute meritocracy, recruiting proven scientific talents wherever they could be found.
By Caltech’s own admission, their Committee on Naming and Recognition (CNR), which recommended that Millikan’s name be scrubbed from the central building on campus, was formed not because new information had come to light, but for the same reason that the rest of the country had been set ablaze:
On May 25, 2020, police officers in Minneapolis, Minnesota, killed George Floyd, a 46-year-old Black man. The tragedy triggered international outrage and protests. In the weeks that followed, Institute leadership received calls for action to improve diversity and inclusion within the Caltech community. Two significant petitions, each with more than 1,000 signatures, demanded the removal from all campus assets and honors of the names of past Institute leaders who had been associated with eugenics and the [Human Betterment Foundation].
Having served on a few collegiate committees myself, I don’t find it at all surprising that the CNR ultimately sided with the petitioners. Committees usually work to find practical solutions, not some abstract truth, and the attitudes of the students who led the protests (“I don’t want to have that constant reminder that the people who built this institution didn’t want me to be there, and didn’t even want me to exist”) are of a sort that would be hard to dismiss without redress.

The CNR’s long report, which is available in full online, covers an emotional response with a fig leaf of rationality. The report’s central claim against Millikan is that he “joined the [Human Betterment Foundation] as a trustee in 1937, at a time when eugenics and its claims for the hereditary nature of human behavior and character had fallen into disrepute within various quarters of the scientific and broader academic community.” Quotes from biological luminaries are cited to support the claim that eugenics, by 1937, was a fringe scientific belief.
Thomas Hales, a mathematician at the University of Pittsburgh, compared claims from the CNR report against the historical record, in an article he posted last September. Hales discovered that some of the biologists cited by the report as opponents of eugenics were in fact supporters of forced sterilization—sometimes in the very same quotes used as evidence against Millikan. Furthermore, Millikan never attended any Foundation meetings, and saw his membership as just another of the charitable causes to which he lent his support.
“Robert Andrews Millikan was not a villain,” wrote Goodstein in his “Defense”—but whether he was or not hardly matters, at this point. Once Caltech issued its judgment against Millikan, it was all but impossible for the American Association of Physics Teachers to continue to bestow its own Millikan Prize. Millikan’s association had been tainted; no longer a household name, and no longer around to fight his own battles, Millikan had become synonymous with scientific racism. It turns out that it’s easier to rename things than to advocate for historical nuance.
