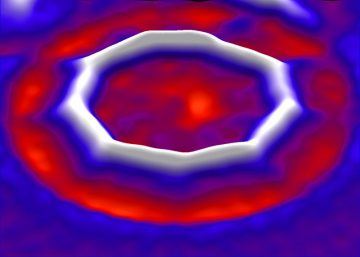
 Long after most chemists had given up trying, a team of researchers has synthesized the first ring-shaped molecule of pure carbon — a circle of 18 atoms. The chemists started with a triangular molecule of carbon and oxygen, which they manipulated with electric currents to create the carbon-18 ring. Initial studies of the properties of the molecule, called a cyclocarbon, suggest that it acts as a semiconductor, which could make similar straight carbon chains useful as molecular-scale electronic components. It is an “absolutely stunning work” that opens up a new field of investigation, says Yoshito Tobe, a chemist at Osaka University in Japan. “Many scientists, including myself, have tried to capture cyclocarbons and determine their molecular structures, but in vain,” Tobe says. The results appear in Science1 on 15 August.
Long after most chemists had given up trying, a team of researchers has synthesized the first ring-shaped molecule of pure carbon — a circle of 18 atoms. The chemists started with a triangular molecule of carbon and oxygen, which they manipulated with electric currents to create the carbon-18 ring. Initial studies of the properties of the molecule, called a cyclocarbon, suggest that it acts as a semiconductor, which could make similar straight carbon chains useful as molecular-scale electronic components. It is an “absolutely stunning work” that opens up a new field of investigation, says Yoshito Tobe, a chemist at Osaka University in Japan. “Many scientists, including myself, have tried to capture cyclocarbons and determine their molecular structures, but in vain,” Tobe says. The results appear in Science1 on 15 August.
Pure carbon comes in several different forms, including diamond, graphite and ‘nanotubes’. Atoms of the element can form chemical bonds with themselves in various configurations: for example, each atom can bind to four neighbours in a pyramid-shaped pattern, as in diamond; or to three, as in the hexagonal patterns that make up the single-atom-thick sheets of graphene. (Such a three-bond pattern is also found in bulk graphite as well as in carbon nanotubes and in the globular molecules called fullerenes.) But carbon can also form bonds with just two nearby atoms. Nobel-prizewinning chemist Roald Hoffmann at Cornell University in Ithaca, New York, and others have long theorized that this would lead to pure chains of carbon atoms. Each atom might form either a double bond on each side — meaning the adjacent atoms share two electrons — or a triple bond on one side and a single bond on the other. Various teams have attempted to synthesize rings or chains based on this pattern.
More here.
