by Ashutosh Jogalekar
I loved chemistry so deeply that I automatically now respond when people want to know how to interest people in science by saying, “Teach them elementary chemistry”. Compared to physics it starts right in the heart of things. —Robert Oppenheimer
How much science can you teach very young children? I have been exploring this question for the past one year or so as I experiment with teaching various kinds of scientific topics to my 3-year-old daughter. She has been generally quite receptive and curious about everything I tell her, and whatever I have failed to teach her is largely a reflection on either the intrinsic difficulties of explaining certain kinds of ideas or my own communication skills.
Chemistry has always been more accessible than physics to laymen, largely because of its colors and explosions and smells and relevance to everyday life; it “starts right in the heart of things”, as Oppenheimer put it. But even at an elemental level it seems easier because of the architectural nature of the subject – atoms which are the building blocks assemble into larger molecules. The basic idea to communicate was to think of atoms as balls that can attach to different numbers of other balls based on what element they represent – one for hydrogen, two for oxygen, three for nitrogen, four for carbon and so on. The combination of atoms into molecules then becomes a simple exercise — just count whether each atom attaches to its right number of neighbors. To make it friendlier, I asked my daughter to see if each atom has the right number of “friends” it holds hands with.
Chris Ferrie’s “Organic Chemistry For Babies” was quite useful for doing this. It has colorful illustrations of the many different shapes – corresponding to different molecules – that atoms can combine into.
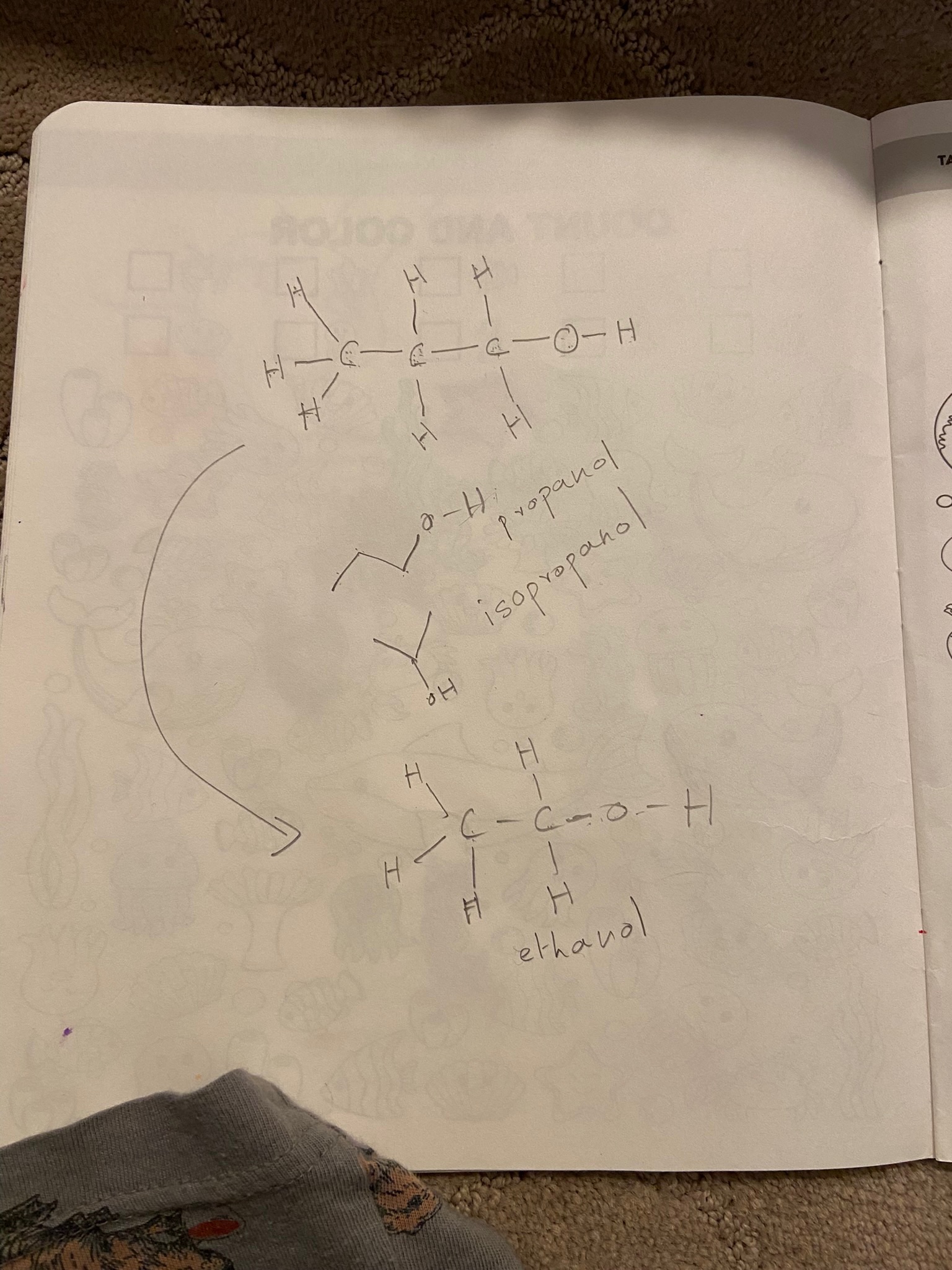
From there it was a logical step to an exercise one morning when we had to use rubbing alcohol (isopropanol) to wipe some color off her tracing book. Knowing the different letter symbols that every element had, it was easy to understand how to write the molecular formula of isopropanol. My daughter could count the number of lines between the different atoms and make sure it was the right number for that particular element.
It also gave me an opportunity to explain isomers – in this case isopropanol and propanol. The simple analogy was with kids holding hands and dancing; isomers are just different arrangements of dance partners.
Once the foundation for understanding elements as different kinds of atoms, each bonding to a different number of other atoms was laid, understanding molecules as combinations of atoms was easy. Carbon dioxide (CO2), water (H2O), and oxygen (O2) were easily drawn and understood. But what’s great about elements and molecules is that once a child understands the infinite combinations that these represent, a world of wonder opens up.
For instance, explaining the process of photosynthesis then became easy: “Green plants use a molecule called chlorophyll to mix up sunlight and water with carbon dioxide to make food for animals and humans and breathe out oxygen.”
Which then logically leads to what this oxygen can do:
“People eat food. This food mixes with oxygen to give them energy to jump up and down and run around. This also makes carbon dioxide which we breathe out, which plants then again breathe in for doing photosynthesis.”
Photosynthesis and respiration explained in one fell swoop as molecules turning into other molecules, with an opportunity to say why we need to care about planting trees.
Even more wonderfully, an understanding of elements gave me a chance to explain to her the process of nucleosynthesis inside stars. This is one of the truly great facts of science, how all our bodies are all literally made inside dying stars like supernovas. But now chemistry turns to physics because you can’t explain why elements are forged in the furnaces inside stars without explaining white dwarfs, neutron stars and black holes.
This isn’t very hard: “Inside the sun, four hydrogen atoms hug each other to form a helium atom and make a lot of heat and light. But gravity is pulling everything together. When the hydrogen is all eaten up, gravity can make the star collapse since there’s nothing to push back against it. In bigger stars even more elements can be formed this way. But in really big stars, gravity finally crushes the star completely. This can happen very fast and leads to a giant explosion which scattered all the elements into space through a giant burp. They travel really far into solar systems like ours where, just once in a while, they make living things like us.”
All elements, that is, except hydrogen which was created in the Big Bang. And gold which is made when two neutron stars crash into each other in an explosion of unimaginable violence. Two other fun and profound facts which are easy for a toddler to appreciate.
Of course, chemistry is an experimental science and so there’s not much joy to it without experiments, which are the next step. And what better experiment to kick things off than one which I did countless times as a kid and which is just unadulterated fun – the mixing of potassium permanganate and glycerol, resulting in a fiery exothermic reaction.
And when all the fun is done, end the day with even more fun, polishing off our knowledge of elements with Tom Lehrer’s famous song which she and I are now practicing.