Heidi Ledford in Nature:
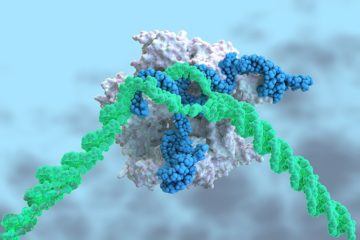 Preliminary results from a landmark clinical trial suggest that CRISPR–Cas9 gene-editing can be deployed directly into the body to treat disease. The study is the first to show that the technique can be safe and effective if the CRISPR–Cas9 components — in this case targeting a protein that is made mainly in the liver — are infused into the bloodstream. In the trial, six people with a rare and fatal condition called transthyretin amyloidosis received a single treatment with the gene-editing therapy. All experienced a drop in the level of a misshapen protein associated with the disease. Those who received the higher of two doses tested saw levels of the protein, called TTR, decline by an average of 87%.
Preliminary results from a landmark clinical trial suggest that CRISPR–Cas9 gene-editing can be deployed directly into the body to treat disease. The study is the first to show that the technique can be safe and effective if the CRISPR–Cas9 components — in this case targeting a protein that is made mainly in the liver — are infused into the bloodstream. In the trial, six people with a rare and fatal condition called transthyretin amyloidosis received a single treatment with the gene-editing therapy. All experienced a drop in the level of a misshapen protein associated with the disease. Those who received the higher of two doses tested saw levels of the protein, called TTR, decline by an average of 87%.
The treatment was developed by Intellia Therapeutics of Cambridge, Massachusetts and Regeneron of Tarrytown, New York. They published the trial results in The New England Journal of Medicine1 and presented them at an online meeting of the Peripheral Nerve Society on 26 June. Previous results from CRISPR–Cas9 clinical trials have suggested that the technique can be used in cells that have been removed from the body. The cells are edited and then reinfused back into study participants. But to be able to edit genes directly in the body would open the door to treating a wider range of diseases. “It’s an important moment for the field,” says Daniel Anderson, a biomedical engineer at the Massachusetts Institute of Technology in Cambridge. “It’s a whole new era of medicine.”
More here.
