Archa Fox in The Scientist:
 I have a clear memory of presenting my initial results about a “failed” protein at a lab meeting with my postdoctoral advisor Angus Lamond at the University of Dundee in Scotland and the rest of his group. It was the summer of 2000, and for the first few months of my postdoc I had been fusing green fluorescent protein with novel proteins that had recently been identified by mass spectrometry as residing in the nucleolus. I engineered HeLa cells to produce copious amounts of these fusion proteins, and watched where they went. Most migrated to the nucleolus, as expected, but one protein steadfastly refused. Instead, it formed nuclear dots that were much smaller than the large and obvious nucleoli. I was really worried about the messiness of this result, but also intrigued. To my relief, instead of being disappointed that the protein was not doing what we had expected, Angus and my lab mates encouraged me to explore it further. The group had access to antibodies against many cellular structures, so I quickly established that these nuclear dots were different from any known nuclear bodies. But having generated much of my data with overexpressed protein, it was critical to make sure that the endogenous form of the protein also localized to the same nuclear dots, and that what I had seen were not simply artifacts of my approach.
I have a clear memory of presenting my initial results about a “failed” protein at a lab meeting with my postdoctoral advisor Angus Lamond at the University of Dundee in Scotland and the rest of his group. It was the summer of 2000, and for the first few months of my postdoc I had been fusing green fluorescent protein with novel proteins that had recently been identified by mass spectrometry as residing in the nucleolus. I engineered HeLa cells to produce copious amounts of these fusion proteins, and watched where they went. Most migrated to the nucleolus, as expected, but one protein steadfastly refused. Instead, it formed nuclear dots that were much smaller than the large and obvious nucleoli. I was really worried about the messiness of this result, but also intrigued. To my relief, instead of being disappointed that the protein was not doing what we had expected, Angus and my lab mates encouraged me to explore it further. The group had access to antibodies against many cellular structures, so I quickly established that these nuclear dots were different from any known nuclear bodies. But having generated much of my data with overexpressed protein, it was critical to make sure that the endogenous form of the protein also localized to the same nuclear dots, and that what I had seen were not simply artifacts of my approach.
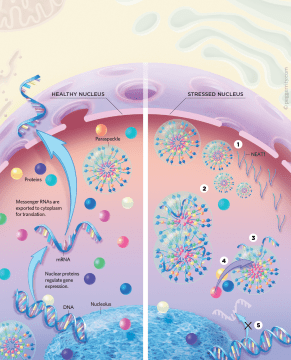
We created an antibody against the protein and incubated it with HeLa cells. It was an incredibly nerve-wracking moment looking down the microscope to see what the antibody had stained. Thankfully, it worked. I was ecstatic when I saw that it had picked out the same small nuclear dots that I had identified with GFP. In 2002, I published a manuscript introducing the scientific community to paraspeckles— orbs of protein and nucleic acid 360 nanometers in diameter, squeezed next to the more famous and larger structures called nuclear speckles. Since then, paraspeckles have become an established part of cell biology; there are more than 250 articles on them, and they have already found their way into some textbooks. We now know they are membraneless organelles seeded by a long noncoding RNA (lncRNA), formed through a well-characterized physical phenomenon known as liquid-liquid phase separation, and composed of numerous proteins and RNA molecules. We also know that they can alter gene regulation when cells get stressed, an important mechanism for maintaining cell homeostasis and one that appears to be disrupted in many diseases.
More here.
