Azra Raza in the Wall Street Journal:
Most patients continue to face excruciating, costly and ineffective treatments. It’s time to shift our focus from fighting the disease in its last stages to finding the very first cells.

I have been studying and treating cancer for 35 years, and here’s what I know about the progress made in that time: There has been far less than it appears. Despite some advances, the treatments for most kinds of cancer continue to be too painful, too damaging, too expensive and too ineffective. The same three methods—surgery, chemotherapy and radiotherapy—have prevailed for a half-century.
Consider acute myeloid leukemia, the bone-marrow malignancy that is my specialty. AML accounts for a third of all leukemia cases. Currently, the average age of diagnosis is 68; roughly 11,000 individuals die annually from the disease. The five-year survival rate for diagnosed adults is 24%, and a bone-marrow transplant increases the odds to 50% at best. These figures have hardly budged since the 1970s.
The overall rate of cancer deaths in the U.S. has fallen by a quarter since its peak in 1991, translating to 2.4 million lives saved—but improved treatments are not the primary reason. Rather, a reduction in smoking and improvements in screening have led to 36% fewer deaths for some of the most common cancers—lung, colorectal, breast and prostate. And for all those gains, overall cancer death rates are not dramatically different from what they were in the 1930s, before they rose along with cigarette use. Meanwhile, cancer drug costs are spiraling out of control, projected to exceed $150 billion by next year. With the newest immunotherapies costing millions, the current cancer-treatment paradigm is fast becoming unsupportable.
What we need now is a paradigm shift. Today, the newest methods generating the most research and expense tend to be focused on treating the worst cases—chasing after the last cancer cells in end-stage patients whose prognoses are the worst. We need instead to commit to anticipating, finding and destroying the first cancer cells. We must reliably detect the faint footprints of cancer at the beginning and stop it in its tracks. Such prevention represents the cheapest, fastest and safest alternative to the terrible, longstanding treatment trio of slash, poison and burn. It’s the most universally applicable way to save lives, and the estimated cost-savings from early diagnosis add up to over $26 billion a year, more than any other new approach can promise.
Earlier detection is also the most humane way to improve cancer outcomes. Status quo treatments—the combination of surgery, chemotherapy and radiation for solid tumors, or chemo and bone marrow transplants for liquid ones—can be brutal and indiscriminate killers. Treatments often leave patients in agony, while providing mere months of added survival. The new immunotherapies can be even more dangerous and harsh. Patients have to be treated in intensive care units, and entire industries are sprouting up just to control the deadly side effects.
I’ve experienced the pain of this situation from the other side of the hospital bed. My own husband, a leading oncologist himself, survived one cancer at the age of 34 and became convinced that he was destined to die young, suspecting every stray blemish of being malignant. When a swollen lymph node appeared in his neck in February 1998, he suspected the worse. At first, we were relieved to find it was only a lymphoma and not a much more terrifying metastatic appearance of his previous cancer.

But the treatments we gave him caused his immune system to collapse. His weight went down to a mere 139 pounds from 210, and his face became disfigured by lesions and paralysis. At our daughter’s eighth birthday party, I found him hiding in a bedroom. “What’s wrong?” I asked? “I did my best to live until her birthday,” he answered. “I need another target, Az.” I managed to say with bogus good cheer, “What about my birthday?” He looked wistfully at me and smiled, “I’m afraid that is too far.” Four months later, he died of sepsis.
I’m not suggesting that the entire field of oncology has failed us. Lymphoma, in fact, is one of the types of cancers for which outcomes have improved, and there are others. In the 1970s, the odds of surviving five years with mediastinal germ-cell tumors—a cancer growing from reproductive cells but which arises in the chest—was only 3%, regardless of the stage; now that ranges from 42% to 76%. Survival rates for patients with melanoma, a cancer of the skin, and multiple myeloma, a cancer of bone marrow plasma cells, also have risen significantly.
Such improvements, however, also planted the seeds of our current misguided approach. The most dramatic results were achieved with treatments developed more than 20 years ago in two blood cancers, each of which was found to be the result of a single cellular abnormality that could be addressed by a single drug. These successes were very welcome, but they had a downside. They seemed to confirm that cancer results from a genetic mutation that can be cured with a “magic bullet.” Enormous resources were invested in the hunt for single mutations in other cancers, which has evolved into a hugely popular medical effort known as “precision oncology.” The idea is to sequence tumors, identify the key mutations responsible for the uncontrolled growth of cells and block their action with a specific drug.
As precision oncology gained steam, a clinical-trial initiative spearheaded by the National Cancer Institute called the Molecular Analysis for Therapy Choice, or MATCH, started in 2015. There were more than 30 arms of this trial, and the results are slowly trickling in. Among the more common tumors tested, “actionable” mutations addressable by existing drugs were found in 15% of cases at best. A bigger disappointment is that even pairing a mutation to a drug did not guarantee results—only a third of the matched patients responded to the treatment, and half of those responses faded within six months. Though the pursuit of precision oncology has by no means folded, expectations have been seriously dialed down.
A woman undergoing chemotherapy, and wearing cooling headgear to prevent hair loss. PHOTO: VÉRONIQUE BURGER/SCIENCE SOURCE
In reality, most common cancers are far more confounding. No wonder that up to 95% of cancer drugs brought to the bedside in clinical trials fail to win FDA approval. And among the other 5%, many hardly deserve their approvals, as their improvements to survival only last a few months and for a fraction of the treated cases. An exception has been the introduction of new immunotherapies like CAR-T (chimeric antigen receptor T cells). These have allowed some hopeless patients with lung cancer, melanoma, lymphoma and acute lymphoblastic leukemia to live years beyond their predicted survival. But there are serious problems with the much-hyped CAR-T. The treatment is available, at a financially prohibitive cost, for very few patients. Worse, it can be physically toxic and is not universally curative.
So what is the solution?
For one thing, all of us in the biomedical sciences need to descend from our high horse and humbly admit where we have been wrong. We have sought to model cancer in petri dishes and mice, seeking out single drugs for simple genetic mutations. But cancer is far too complex a problem to be solved with such reductionism. We have not made much progress in the past 50 years and won’t advance much more in another 50 if we insist on the same-old same-old.
The idea of early detection as the best, most effective approach is not new. As far back as 1907, the British physician Charles Childe, author of “The Control of a Scourge,” observed, “Cancer itself is not incurable … it is the delay that makes it so,” and pushed for a public campaign for early intervention. The American Society for the Control of Cancer promoted early detection drives from the 1930s to 1950s through a “Women’s Field Army” with the slogan, “Delay Kills.”
Cancer research has been promising hope and delivering disappointments for a half-century.
Current screening tests have proven the potential for a major shift in focus, but they are far from perfect. Monitoring methods such as mammography, colonoscopy, Pap smears and testing for prostate specific antigen (PSA) have been in use for decades, but not all are uniformly successful. Some lesions progress so slowly that a patient is more likely to die of something else, while some dangerous tumors still are not detectable before it is too late to cure them.
Despite the problems, several large studies have found reduced breast cancer deaths for women screened by mammograms, and death rates from colorectal cancers and cervical cancers, which proceed in a stepwise manner from inception to dangerous disease, also have improved with screening. But the same is not true for lung cancer, for example. Three randomized trials failed to show a reduction in lung cancer deaths from screening. Much of the treatment given to the patients was at best unnecessary, and some of it proved actually harmful.
The quest for me and many others in the field is to move beyond such annual tests and develop technology that would provide continuous machine-monitored screening of the human body. That in turn would enable newly effective treatments to head off cancer in early stages—for instance, quickly zapping a small budding group of malignant cells with a laser without the need for prolonged courses of radiation and chemotherapy. And we already have a number of drugs that work well only in the early stages of cancer, including for leukemia.
One urgent need in this effort is to define “biomarkers” of cancerous cells. These can be almost anything—an unusual protein, a strand of genetic material or some compound—that the cancer cells release into our bodies. The National Cancer Institute is already supporting several large initiatives with the hope that such biomarkers will not only provide the earliest footprints of cancer but also help to separate aggressive tumors from non-life-threatening ones.
A good example is the work of Dr. Bert Vogelstein’s research group at the Johns Hopkins School of Medicine. In 2018, they developed CancerSEEK, a blood test that measures eight cancer proteins and 16 genetic mutations from DNA circulating in the blood. This “liquid biopsy” correctly spotted the presence of cancer in 70% of samples obtained from patients with eight types of malignancies. Nor were they simply finding new ways to do old things. Five of the cancers in question (ovary, liver, stomach, pancreas and esophagus) have no currently available screening methods.
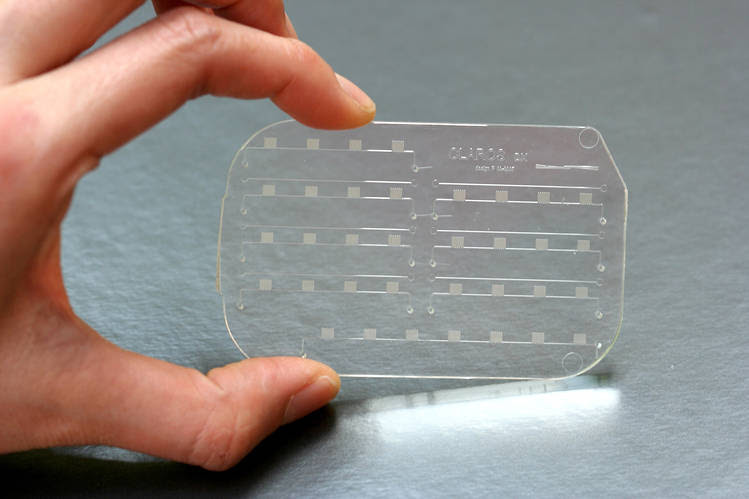
The mChip, developed at Columbia University, has been approved by the FDA to detect one cancer biomarker so far. PHOTO: SIA LAB AT COLUMBIA ENGINEERING AND OPKO HEALTH
Equally promising are some of the imaging and wearable devices being developed at centers devoted to early cancer detection. At Stanford University’s Canary Center, Dr. Sanjiv Gambhir’s lab has managed to modify a specific type of immune cell to patrol the body for the presence of malignant cells and to send red alerts through the blood and urine if they are detected. His laboratory is also developing a “smart toilet” to sample stool for the DNA of mutated tumors indicating early colorectal cancer. And an ongoing clinical trial there is testing a “smart bra” fitted with integrated thermal sensors to detect circadian temperature changes associated with early-stage breast cancers.
My own efforts in early detection began in 1984, when I sensed that my cancer specialty, AML, would prove difficult to cure in my lifetime. Sadly, so far, I have been proved right. We are still using the same two drugs to treat AML that we were using in 1977, with the same dreadful results. Back then, I pinned my hopes on finding this leukemia in its pre-leukemic stage, and I needed human cells to study. Thus began my tissue repository, which contains thousands of samples drawn from my patients over the past 35 years as they progressed through the natural history of their diseases. Though we have regularly studied selected samples from this precious bank of cells and made useful observations, we lacked the technology to analyze enormous batches of them. Recent improvements in genomics and mass spectrometry have now made it practical and economical to comprehensively examine tissue to find new biomarkers.
Sometimes, rather than improving existing methods, a drastic change in strategy is essential. Our idea is to develop a radically different set of tools for early cancer detection, by identifying those novel biomarkers and developing imaging and implantable devices to provide persistent monitoring of healthy bodies. We have high hopes for a device that has been dubbed the mChip, developed by Dr. Samuel Sia, a biomedical engineer at Columbia University. It uses microfluidics to perform lab-quality measurement of a cancer marker from a small amount of blood in a test that can be performed at home. The company OPKO recently obtained FDA approval for the mChip to detect one biomarker, for early-stage prostate cancer. Once we identify the right biomarkers, the mChip technology could be equally effective in sighting the first leukemia cell.
Such sporadic efforts by a few researchers will take far too long, however, to yield the needed answers. We need to go further and redirect intellectual and financial resources from the usual grant proposals to early detection using actual human samples. By posing exciting challenges to competitive scientists, progress can be accelerated dramatically.
What’s missing from today’s discussion of cancer is the admission that current strategies have failed and we need to take a 180-degree turn. We now invest a lot of effort into finding minimal residual disease. Why not apply the same rigor and focus to finding minimal initial disease? Cancer research has been promising hope and delivering disappointments for a half-century. Instead of letting cancer grow into its end-stage monstrosity, let us assemble our resources to pre-empt that battle and strike instead at cancer’s root: the first cells.
This essay is adapted from Dr. Raza’s new book, “The First Cell and the Human Costs of Pursuing Cancer to the Last,” which will be published on Oct. 15 by Basic Books. She is the Chan Soon-Shiong Professor of Medicine and director of the MDS Center at Columbia University.
Editor’s Note: This article is published here in its entirety with the explicit permission of the Wall Street Journal.
