Kerry Grens in The Scientist:
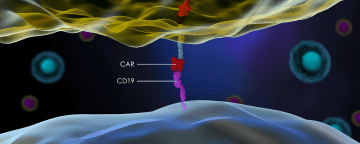 Despite the recent approval of two cancer therapies that use CAR T cells to treat lymphoma, 25 percent of eligible patients still choose to enter clinical trials instead of undergoing the available treatments. That’s according to insurance claims analyzed by health care consulting firm Vizient, Reuters reports. Cost may be the driving factor behind patients’ decisions to forgo CAR T therapies already on the market for those still in clinical trials. Approved CAR T interventions Kymriah and Yescarta carry price tags in the hundreds of thousands of dollars, while experimental treatments are typically covered by the trials’ sponsors. “Inadequate inpatient reimbursement, especially for Medicare patients, can be a significant deterrent for hospitals to use commercially approved CAR-Ts,” Jennifer Tedaldi, associate principal at consulting firm ZS Associates, tells Reuters.
Despite the recent approval of two cancer therapies that use CAR T cells to treat lymphoma, 25 percent of eligible patients still choose to enter clinical trials instead of undergoing the available treatments. That’s according to insurance claims analyzed by health care consulting firm Vizient, Reuters reports. Cost may be the driving factor behind patients’ decisions to forgo CAR T therapies already on the market for those still in clinical trials. Approved CAR T interventions Kymriah and Yescarta carry price tags in the hundreds of thousands of dollars, while experimental treatments are typically covered by the trials’ sponsors. “Inadequate inpatient reimbursement, especially for Medicare patients, can be a significant deterrent for hospitals to use commercially approved CAR-Ts,” Jennifer Tedaldi, associate principal at consulting firm ZS Associates, tells Reuters.
CAR T therapies involve extracting a patient’s T cells, engineering them to contain chimeric antigen receptors (CARs), and infusing the cells back into the patient to seek out and destroy cells bearing antigens complementary to the CARs on their surface. The US Food and Drug Administration approved Kymriah in August 2017 and Yescarta two months later, in October. From May 2017 until December 2018, according to Vizient’s data, 900 patients at 58 hospitals in the US received a CAR T intervention, and 25 percent of those patients did so through a clinical trial. Because this time period stretches back a few months before the cell therapies hit the market, the data include clinical trial patients who may have received the treatments pre-approval. Medical bills for the patients on experimental cell therapies were half as much as those for patients receiving the approved treatments.
More here.
