Marcela Maus in Nature:
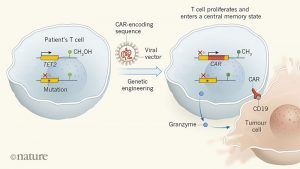 The use of genetically engineered immune cells to target tumours is one of the most exciting current developments in cancer treatment. In this approach, T cells are taken from a patient and modified in vitro by inserting an engineered version of a gene that encodes a receptor protein. The receptor, known as a chimaeric antigen receptors (CAR), directs the engineered cell, called a CAR T cell, to the patient’s tumour when the cell is transferred back into the body. This therapy can be highly effective for tumours that express the protein CD19, such as B-cell acute leukaemias1,2 and large-cell lymphomas3,4. However, some people do not respond to CAR T cells, and efforts to optimize this therapy are ongoing. In a paper in Nature, Fraietta et al.5 report the fortuitous identification of a gene that positively affected one person’s response to treatment with CAR T cells.
The use of genetically engineered immune cells to target tumours is one of the most exciting current developments in cancer treatment. In this approach, T cells are taken from a patient and modified in vitro by inserting an engineered version of a gene that encodes a receptor protein. The receptor, known as a chimaeric antigen receptors (CAR), directs the engineered cell, called a CAR T cell, to the patient’s tumour when the cell is transferred back into the body. This therapy can be highly effective for tumours that express the protein CD19, such as B-cell acute leukaemias1,2 and large-cell lymphomas3,4. However, some people do not respond to CAR T cells, and efforts to optimize this therapy are ongoing. In a paper in Nature, Fraietta et al.5 report the fortuitous identification of a gene that positively affected one person’s response to treatment with CAR T cells.
Therapies involving engineered immune cells use viral vectors based on retroviruses or lentiviruses to insert a DNA sequence, such as one encoding a CAR, into a person’s T cells. However, given that there is no control over where the sequence inserts into the genome, it is possible that the engineered gene could insert at a location that disrupts another, important gene. In the early 2000s, a clinical trial6 enrolled people with immunodeficiencies arising from the lack of a functional copy of a particular immune gene. The trial used viral vectors to insert a wild-type copy of this gene into their stem cells. Unfortunately, however, several people developed uncontrolled T-cell proliferation that evolved into T-cell leukaemia. This event was linked7 to the gene inserting within the sequence of the LMO2gene, disrupting the normal regulation of LMO2.
The pattern of genomic integration sites for various viral vectors has been found to be specific for a given combination of vector and cell type8. In a study of people who had T cells modified using retroviral vectors, the integration events were not implicated as the cause of any cancers9. Lentiviral vectors integrate randomly into the genome but tend to preferentially locate at sites of transcriptionally active genes10. Although random integration is generally thought to be safe, any disruption of the genome nevertheless confers a risk of unwanted consequences.
More here.
