Donald Ingber in The Scientist:
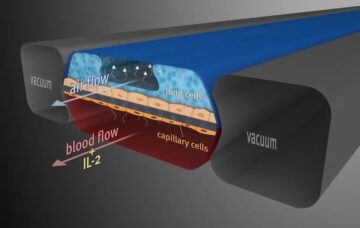 In April of this year, the Food and Drug Administration (FDA) announced a new roadmap that aims to replace animal testing in the development of new drugs with more human-relevant methods. The goal is to improve drug safety, accelerate the evaluation process, shorten drug development timelines, reduce costs, and spare animal lives. The FDA aims to make animal testing the exception rather than the norm within three to five years. Is this possible?
In April of this year, the Food and Drug Administration (FDA) announced a new roadmap that aims to replace animal testing in the development of new drugs with more human-relevant methods. The goal is to improve drug safety, accelerate the evaluation process, shorten drug development timelines, reduce costs, and spare animal lives. The FDA aims to make animal testing the exception rather than the norm within three to five years. Is this possible?
The FDA has required animal testing of new drugs since the 1930s after more than 125 American adults and children died after ingesting an antibiotic elixir that mistakenly contained the poison found in antifreeze—diethylene glycol—which was then thought to be just a sweetening agent. Animal testing remains the mainstay of drug evaluations by the FDA, and it undoubtedly has helped prevent other potentially dangerous chemicals from reaching patients. However, on the flip side, the results of drug tests in animals fail to predict future responses in humans more than 90 percent of the time.1 It is also likely that many drugs that could have been safe and effective in humans never received approval because they were found to be toxic in early animal studies. Aspirin is a great example; we are all lucky because it was first marketed before 1900.
More here.
Enjoying the content on 3QD? Help keep us going by donating now.
