Mizrahi and Barry in Nature:
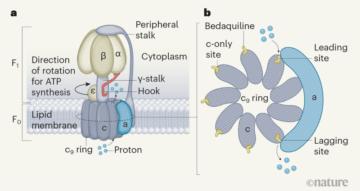 The 2017 Nobel Prize in Chemistry was awarded for the development of an imaging method called cryo-electron microscopy. On bestowing the prize, the Royal Swedish Academy of Sciences stated that this technique has “moved biochemistry into a new era”. Writing in Nature, Guo et al.1 provide a compelling glimpse into this new age. The authors’ work reveals how a drug called bedaquiline, which has revolutionized the treatment of drug-resistant tuberculosis2,3, interacts with its target.
The 2017 Nobel Prize in Chemistry was awarded for the development of an imaging method called cryo-electron microscopy. On bestowing the prize, the Royal Swedish Academy of Sciences stated that this technique has “moved biochemistry into a new era”. Writing in Nature, Guo et al.1 provide a compelling glimpse into this new age. The authors’ work reveals how a drug called bedaquiline, which has revolutionized the treatment of drug-resistant tuberculosis2,3, interacts with its target.
The drug binds to the ATP synthase enzyme of the microorganism Mycobacterium tuberculosis that causes tuberculosis4. Adding to a rapidly growing body of work elucidating the structure of ATP synthases by cryo-electron microscopy5,6, the details presented by Guo and colleagues — and particularly the videos (see Supplementary Videos 1 and 2 of ref. 1) generated from their structural data — are breathtaking in their ability to reveal how this macromolecular machine works. Moreover, the authors show how the drug binds to the enzyme and disrupts its synthesis of the molecule ATP, providing crucial information that had eluded detection through other, more conventional structural and biochemical investigative techniques.
More here.
