by Carol A Westbrook

The Supreme Court is poised to make another landmark decision this year, when it determines if it will uphold a Texas Federal court’s ruling that invalidates the FDA’s (U.S. Food and Drug Administration’s) updated labeling of the abortion pill mifepristone (pronounced mi-ˈfe-pri’-stōn) , brand name Mifeprex (Fig 1). Not only will this ruling have a significant impact on abortions in the US, it will also determine whether the Supreme Court (Fig 2) has the power to modify or nullify an FDA ruling. But before we delve any further into this debate, let’s review the action of this drug on the biology of the female reproductive system.

In the early part of a woman’s monthly cycle, her levels of the hormone progesterone rise, and this causes the lining of the uterus to thicken and increases its blood supply, converting it into a state that can support a fetus. After unprotected sex, sperm are deposited in the vagina, and they begin to travel up the fallopian tubes; at the same time, an egg is released from the ovary and travels down the fallopian tube. (Fig 3) When sperm and egg unite, conception occurs. Interestingly, the date of conception does not mark the start of the pregnancy; pregnancy is actually counted from the beginning of a woman’s monthly cycle, two weeks prior to conception. The total length of a pregnancy is usually 40 weeks, or 9 months.
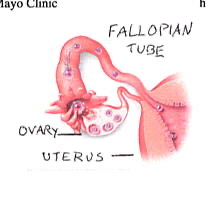
The fertilized egg begins to divide into a small ball of cells, which travel down the fallopian tube toward the uterus. After it reaches the inside of the uterus, the ball of cells stops and burrows into the lining of the uterus; this is called “implantation.” Implantation is where the “Morning After Pill” (Plan B) has its effects, because it opposes the action of progesterone on the lining of the uterus, preventing or reversing implantation.
In an uninterrupted pregnancy, the ball of cells from the fertilized egg begins to elongate and curve into a “C” shape, while the outer cells develop into the placenta. Early in week 6 of the pregnancy, a furrow along the back—the neural tube– begins to close; these cells will become the spinal cord and the brain. The head can now be seen.
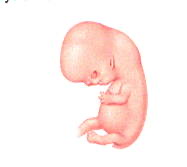
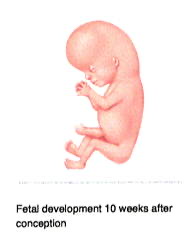
By week 7 (Fig 4), other recognizable features begin to develop, including the head and eyes, and the arm and leg buds. Although the heart has not yet completely formed, the cells that will make it up have begun to beat in unison. The coordinated beating of the heart cells does not mean that a person or a soul has been created, however, because heart cells will also beat in unison if they are grown in the laboratory in petri dishes. This tube of beating cells within the embryo will soon fold into a heart and by week 8, a heartbeat can usually be heard. Additionally, facial features become recognizable, including early eyes, nose, and ears. The fetus at this time is about ½ inch in length from crown to rump. By week 10 (Fig 5) the head makes up about half of the embryo’s length, and finer details like fingers and genitalia can be clearly seen. By week 11 the embryo is officially called a fetus. Week 12 marks the end of the first trimester; the fetus is well-established within the uterus, with an umbilical cord connected to a placenta. The pregnancy is one-third complete.
A mifepristone abortion, also called a medical abortion, actually requires 2 drugs given in succession. The first drug is mifepristone, which inhibits the action of progesterone. After 24-48 hours misoprostol is given. This second drug which causes the uterus to contract and expel its contents. (In some situations, misoprostol alone is used to induce abortion, though the chance of success if much greater when preceded by mifepristone.) Over the next few days the abortion occurs, there is a variable amount of bleeding, and the fetus is expelled. Two weeks after mifepristone is given, the woman returns to the clinic for a post-treatment exam to confirm that all of the uterus contents have been expelled, and that the woman is healthy without any serious problems.
An outpatient medical abortion is generally used only up until the end of the first trimester (the 12th week). Although the mifepristone-misoprostol combination can also induce abortion in the second semester (12-20 weeks), it is generally not recommended for outpatient administrations. That is because second trimester abortions are longer and involve more bleeding, because the fetus is larger, as is the placenta, and both will have to be dislodged from the uterus. Second trimester terminations are recommended to take place in a health care facility where blood transfusion and emergency surgery are available.
The way the abortion pill is to be administered is spelled out in the FDA labelling—the doses used, who can administer, how many clinic visits are needed, and so on. Since the initial approval in 2000, clinicians have modified the way this is done, making it less expensive, with fewer clinic visits and more involvement of advanced practitioners such as mid-wives and nurse specialists. Although off-label use of drugs is generally accepted in the United States, many clinicians see FDA labels as guides to appropriate and legally defensible clinical practice. First and foremost, the label update reaffirms that medication abortion is very safe and highly effective. It also has the potential to increase access to medication abortion in this country. For that reason, the FDA saw fit to update the labelling to become more in alignment with current medical practice, which has been shown to be safe and effective.
In March 2016, the FDA approved updated labeling for Mifepristone, which contained several significant changes to the original label. These are shown in the table below. The most significant change is an increase in eligibility from 49 days (7 weeks) to 70 days (10 weeks). This may seem like a minor change, but as we saw above, the fetus has had significant time to grow and develop more recognizable human features, and most importantly, to develop a heart with a real heartbeat! The other changes bring the procedure more in line with current practices in today’s health care system, such as the use of providers who are not physicians, and dropping the requirement that the follow-ups had to be done in the clinic, whereas now they can be done using telemedicine. Decreasing the number of in-person visits and decreasing the dose of mifepristone lowered the cost of the procedure.
| OLD LABELING | NEW LABELING |
| Use through 49 days’ gestation | Use through 70 days’ gestation |
| Day 1: Mifepristone 600 mg (3 tablets) orally | Day 1: Mifepristone 200 mg (1 tablet) orally |
| Day 3: Misoprostol 400 µg (2 tablets) orally at healthcare provider facility | 24–48 hours after mifepristone dose: Misoprostol 800 µg (4 tablets) buccally (placed in the cheek); home administration allowed |
| Post-treatment examination: Patients return to provider 14 days after taking mifepristone | Post-treatment assessment: Patients are assessed 7–14 days after taking mifepristone; not necessarily an in-person clinic visit |
| Prescriber: By or under the supervision of a physician who is authorized to prescribe the drug | Prescriber: By or under the supervision of a healthcare provider who is authorized to prescribe the drug |
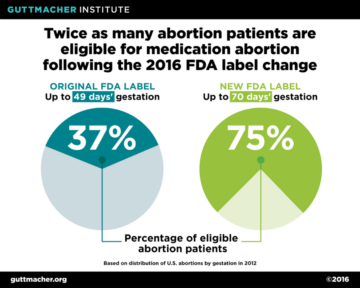
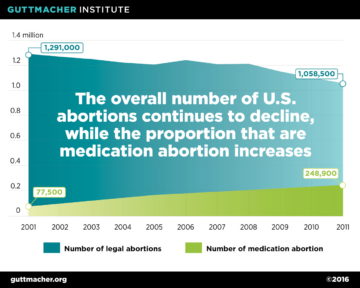
The updated labelling has significant public health implications. A study by the Guttmacher Institute estimated that, following the 2016 labeling changes, twice as many abortion patients would qualify for medication abortion. Under the original protocol, just 37 percent of all U.S. abortions were eligible for mifepristone, but as a result of the updated protocol, the proportion of all abortions now eligible has doubled to 75 percent. (Fig. 6) This is in spite of the fact that the total number of abortions has decreased (Fig 7).
Anti-abortion organizations recognized that these labeling changes make abortion easier and less expensive to get, and took their case to Federal courts to seek an injunction or reversal of the new policy. On April 7, 2023, a federal judge in Texas, Judge Mathew Kacsmaryk, ruled to reverse the approval of the abortion pill, in the Alliance for Hippocratic Medicine v. United States FDA, filed on January 13, 2023. Kacsmaryk’s ruling was a preliminary injunction, deeming the FDA approval of mifepristone in 2000 invalid.
The new FDA ruling on Mifepristone required additional authorization for non-MD providers in order to dispense the pill, so-called REMS requirement, A Risk Evaluation and Mitigation Strategy, or REMS, is a drug safety program that the FDA can require for certain medications with serious safety concerns. On February 24, 2023, 12 states filed a lawsuit against the FDA to ease REMS restrictions enforced for mifepristone; this lawsuit addresses concerns that the current REMS is too restrictive, considering the medication’s safety profile.
Judge Thomas O. Rice of the Eastern District of Washington ruled that access to abortion pills must remain available in 17 states and Washington, DC, without additional restrictions. This injunction directly contradicts Judge Kacsmaryk’s ruling.
Kacsmaryk’s case marks the first lawsuit where a judge decided to overrule FDA approval. If the ruling is enforced, the entire nation will be unable to access mifepristone. Furthermore, it will have widespread implications on FDA approval for any number of medications . Further litigation will likely be required to determine which stands, and the Supreme Court will have to intervene.
The upcoming year , 2024, should be a very interesting one as the pro- and anti-abortion coalitions face off in the courts, the FDA’s evaluation of medical abortions gets resolved, and the role of the Supreme Court in regulating the FDA is clarified.
